Facts in the field of stereochemistry prove the need for a Creator. Against all odds, billions of proteins in our bodies have the same chemical “handedness”!

Enantiomers are called chiral—from the Greek word for hand—because they have mirror-image right-hand and left-hand forms. Even the three-dimensional orientation of the molecules of life gives evidence of a Creator!
The apostle Paul said that God reveals Himself in the creation. He wrote, “For since the creation of the world His invisible attributes are clearly seen, being understood by the things that are made” (Romans 1:20).
The only real alternatives for explaining the existence of matter and life are evolution or God.
Evolutionists have claimed that the building blocks of life can be formed by random natural processes. (Consider the famous Miller-Urey experiment that produced some amino acids.) But, beyond the incredible odds against all the necessary parts forming and assembling themselves mindlessly into something much more complex than a jetliner, there is another problem for evolution.
Even the three-dimensional orientation of the molecules of life gives evidence of a Creator!
Chemical complexity
We believe God created atoms, molecules and all the laws that govern reactions. He created the science of chemistry.
Within the study of stereochemistry (the study of molecular structure in three dimensions) is the study of stereoisomerism. Isomers in general are chemical compounds with the same chemical formula but that differ in chemical structure. Ethyl alcohol and methyl ether consist of the same atoms in the same proportion and thus have the same atomic weights and the same formulas—C2H6O—but they differ in structure (see Diagram 1).
Diagram 1. Examples of compounds that are isomers. The structure of ethanol (C2H6O) is on the left and the structure of dimethyl ether (C2H6O) is on the right. Images created using Maestro by Schrödinger, LLC, 2014.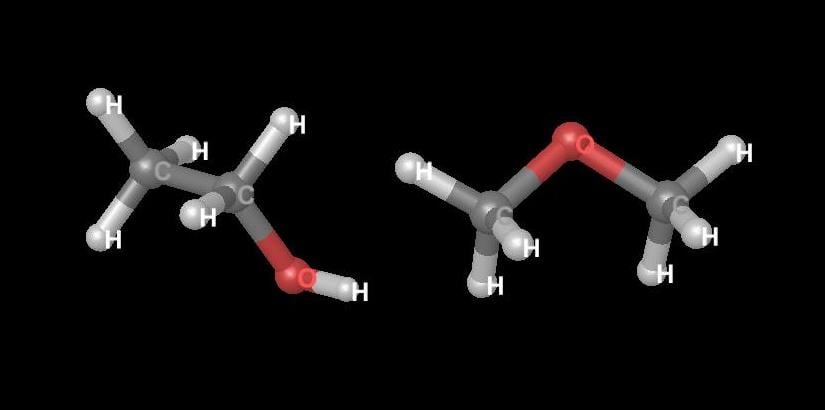
And getting even more specific, there is one type of stereoisomer that is important in the proof of God’s existence—enantiomers. Enantiomers come in two mirror-image forms, each of which is equally likely to form in nature. But, against all odds, usually only one enantiomer of a pair occurs in plants and animal tissues and systems. As we will see, the existence of only one enantiomer of a pair is impossible in nature if all of these substances developed by random chance as evolution claims.
How organic chemistry convinced me of the Creator
While studying organic chemistry in my third year at pharmacy school, I also began studying the Bible. I saw that in order to follow the teachings of God in the Bible, I would have to make some major changes in my thinking and living. But before making these changes, I wanted to find real proof of God’s existence.
I found that proof in an unexpected place.
I had already learned that, in simple terms, it takes a pure enantiomer to produce a pure enantiomer (i.e., it takes one to produce one). The question arose: From where did the first pure enantiomer originate?
(Note that scientists use several words to describe enantiomers. They are called chiral—from the Greek word for hand—because they have mirror-image right-hand and left-hand forms. They are also called optically active since they can be detected using polarized light that is rotated to the right or left when passing through the molecules.)
While studying stereoisomerism I came across the following paragraph in my textbook:
“Some optically active compounds are obtained from natural sources since living organisms usually produce only one enantiomer of a pair. …
I understood that the existence of just one enantiomer of a pair in plants and animals in nature is impossible by natural processes alone. They can only exist as they do as the result of the creative work of God.
“Indeed, we deal with optically active substances to an extent that we may not realize. We eat optically active bread and optically active meat, live in houses, wear clothes, and read books made of optically active cellulose. The proteins that make up our muscles and other tissues, the glycogen in our liver and in our blood, the enzymes and hormones that enable us to grow, and that regulate our bodily processes—all these are optically active. Naturally occurring compounds are optically active because the enzymes that bring about their formation—and often the raw materials from which they are made—are themselves optically active. As to the origin of the optically active enzymes, we can only speculate” (ibid., p. 231, emphasis added).
Man does not know where the first optically active compounds originated, because he does not recognize God as the Creator. In the margin of my textbook by this paragraph I put a big asterisk and wrote EVIDENCE OF GOD’S CREATION.
Why did I write this? I understood that the existence of just one enantiomer of a pair in plants and animals in nature is impossible by natural processes alone. They can only exist as they do as the result of the creative work of God. The proof of this fact is the purpose of this article.
Mirror images
Enantiomers are stereoisomers whose molecular structures (how the atoms are arranged in space) are mirror images of each other. (See again Diagram 2, which shows the two enantiomers of chloroiodomethanesulfonic acid.)
Diagram 2. The mirror images of chloroiodomethanesulfonic acid. The structure on the left is the left-handed enantiomer and the structure on the right is the right-handed enantiomer.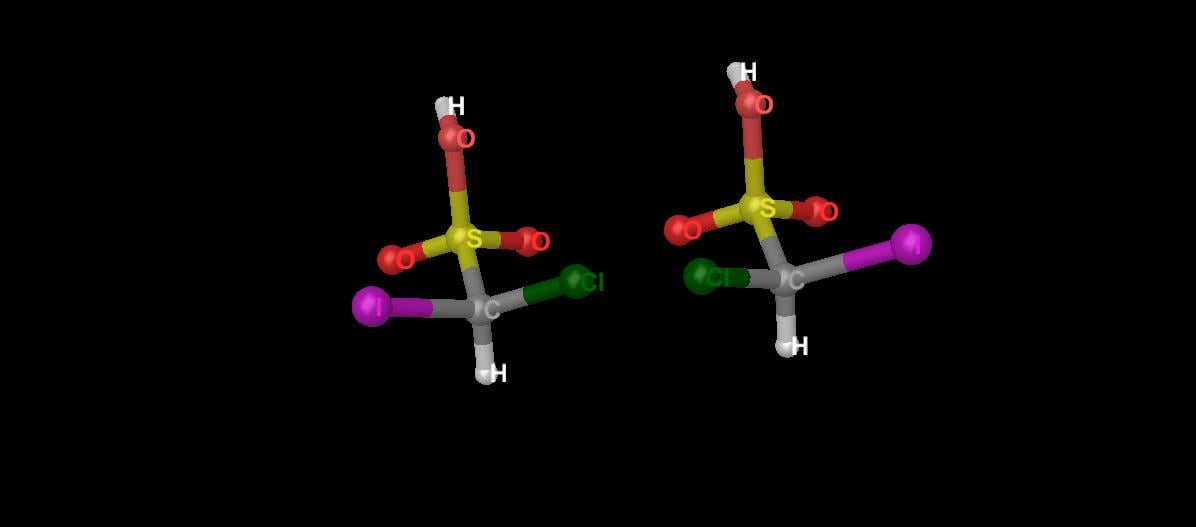
By definition, enantiomers are mirror images of each other. They have the same formulas; but, being mirror images of each other, some of their atoms are not superimposable when the molecules are oriented in the same direction.
In Diagram 2 above, when the two molecules are oriented in the same direction, the chlorine atoms would be superimposed with the iodine atoms. The unique feature of enantiomers is that they all have mirror-image molecules.
Production of enantiomers
When enantiomers are made randomly from raw materials, the product is always a 50-50 mixture of the two possible enantiomers of each pair. This is due to the 50-50 chance that an added atom or functional group (radical) would attach to either the left-hand or right-hand side of the target carbon atom since the target area is spatially flat.
This pair of enantiomers (in a 50-50 mixture) cannot be separated into a 100-percent pure product of either enantiomer by ordinary means since the only physical property that differentiates the two molecules is how each affects plane-polarized light (optical activity).
A pure solution consisting of only a single enantiomer is optically active. It will rotate plane-polarized light either to the right or left by a certain degree unique to that pair. The amount of rotation is the same. The difference between the two enantiomers in each pair is the direction of the rotation—right for one and left for the other.
Other physical properties of an enantiomer pair are identical. As a result, they cannot be separated by most normal physical means. The two molecules have the same boiling points, so they cannot be separated by fractional distillation. They have the same solubility in a given solvent, so they cannot be separated by fractional crystallization. And, since they are held equally strongly by a given absorbent, they cannot be separated by chromatography.
“The separation of a racemic modification [50-50 mixture] into enantiomers … is therefore a special kind of job, and requires a special kind of approach” (ibid., p. 83).
These special methods require the use of a pure enantiomer (which only comes from living things in nature) or intelligent intervention by human beings. Without intelligent intervention, the only way a mixture can be separated into pure enantiomers is through the use of optically active reagents. We must have one to produce one.
Yet we find pure enantiomers in abundance in living things. These could only exist if they were created by God. They cannot exist as a result of the random chance formation of molecules from atoms that evolution requires. Any random chance combination of raw materials would only produce a 50-50 mixture.
In spite of that, in living things, 100-percent pure enantiomers are everywhere. In fact, in living things the usual situation is that only one enantiomer of a pair is found.
Why these impossible but necessary pure enantiomers are vital for life
Even though the mirror-image molecules have the same composition, they have much different effects on living things. The other enantiomer, when produced, is found to be useless or even harmful. “Despite their close similarity, one isomer of such a pair may serve as a nourishing food, or as an antibiotic, or as a powerful heart stimulant, and the other may be useless” (ibid., p. 70).
“In biological systems … such stereochemical specificity is the rule rather than the exception, since the all-important catalysts, enzymes, and most of the compounds they work on, are optically active. The sugar (+)-glucose plays a unique role in animal metabolism and is the basis of a multimillion dollar fermentation industry; yet (–)-glucose is neither metabolized by animals nor fermented by yeasts. When the mold Penicillium glaucum feeds on a mixture of enantiomeric tartaric acids, it consumes only the (+)-enantiomer and leaves (–)-tartaric acid behind. The hormonal activity of (–)-adrenaline is many times that of its enantiomer; only one stereoisomer of chloromycetin is an antibiotic. (+)-Ephedrine not only has no activity as a drug, but actually interferes with the action of its enantiomer. Among amino acids, only one asparagine and one leucine are sweet, and only one glutamic acid enhances the flavor of food” (ibid., p. 82).
As Charles McCombs, who has a Ph.D. in organic chemistry, explains, evolution has deep problems with chirality:
“As nucleotide molecules come together to form the structure of DNA, they develop a twist that forms the double helix structure of DNA. DNA develops a twist in the chain because each component contains chirality or handedness. It is this handedness that gives DNA the spiral shaped helical structure. If one molecule in the DNA structure had the wrong chirality, DNA would not exist in the double helix form, and DNA would not function properly. The entire replication process would be derailed like a train on bad railroad tracks.
“In order for DNA evolution to work, billions of molecules within our body would have to be generated with the ‘R’ [right-handed] configuration all at the same time, without error. If it is impossible for one nucleotide to be formed with chirality, how much less likely would it be for billions of nucleotides to come together exactly at the same time, and all of them be formed with the same chirality? If evolution cannot provide a mechanism that forms one product with chirality, how can it explain the formation of two products of opposite chirality?” (“Evolution Hopes You Don’t Know Chemistry: The Problem With Chirality,” http://www.icr.org/article/evolution-hopes-you-dont-know-chemistry-problem-wi/).
The conclusion: The tissues and systems found in living things are filled with 100-percent pure enantiomers that do not randomly form in a pure state in nature. The first 100-percent pure enantiomers must have been created by God.
Read more about creation and evolution in our other articles on the subject, starting with “Intelligent Design: Can Science Answer the Question, Does God Exist?”





